Introduction: Rilzabrutinib is a potent oral, reversible Bruton tyrosine kinase inhibitor that can treat hematological autoimmune diseases through multiple putative mechanisms of action: (1) inhibition of B-cell activation, (2) interruption of antibody-coated cell phagocytosis by FcϒR in spleen and liver, and (3) induce sustained anti-inflammatory effects (Langrish J Immunol 2021). Preliminary evidence showed that rilzabrutinib treatment resulted in rapid and durable platelet responses with a favorable safety profile in previously treated patients with immune thrombocytopenia (ITP) as studied in part A of a phase 1/2 clinical study (LUNA 2; Kuter N Engl J Med 2022). This abstract summarizes the results of part B that focused on the durability of response with rilzabrutinib in relapsed ITP patients.
Methods: Part B of the multicenter, open-label, phase 1/2 study evaluated the efficacy and safety of rilzabrutinib 400 mg bid in patients with relapsed ITP (NCT03395210). Adult patients aged 18-80 y were eligible with ≥2 baseline platelet counts <30x10 9/L no less than 7 days apart in the 15 days before the first dose. Eligible patients were required to have a past response (achievement of platelet count ≥50x10 9/L) to intravenous immunoglobulin (IVIg)/anti-D or corticosteroid (CS) that was not sustained and failed ≥1 other ITP therapy (that was not IVIg or CS). Stable doses of concomitant CS/thrombopoietin receptor agonists (TPO-RA) were allowed with rilzabrutinib. The primary endpoints for part B were safety and durable platelet response defined as platelet counts ≥50x10 9/L on ≥8 of the last 12 weeks of rilzabrutinib without rescue medication. Patients completing 24 weeks of rilzabrutinib with platelet counts ≥50x10 9/L or ≥30x10 9/L and doubling from baseline in ≥4 of the last 8 weeks of treatment without rescue medication could continue rilzabrutinib in the long-term extension (LTE) period.
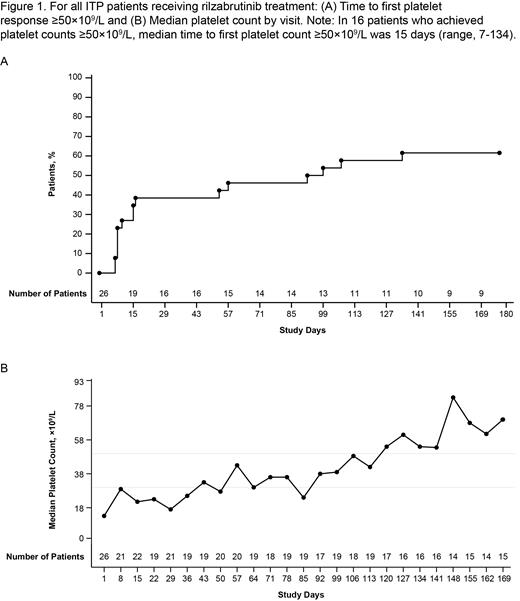
Results: At baseline, 26 enrolled patients had a median age of 57 y (range, 20-75), 62% were female, and median baseline platelet count was 13x10 9/L (range, 2-24x10 9/L). Patients had a median duration of ITP of 10.3 y (range, 0.7-48.2) and had received a median of 6 prior unique ITP therapies (range, 3-19; 46% splenectomy). Seventeen patients (65%) received concomitant non-rescue CS and/or TPO-RA. Nine patients (35%; 95% CI, 17%-56%) achieved the primary endpoint of durable platelet response. Approximately 25% of patients achieved platelet counts ≥50x10 9/L by day 15 of rilzabrutinib treatment (Figure 1A). In 16 patients who achieved platelet counts ≥50x10 9/L, median time to first platelet count ≥50x10 9/L was 15 days (range, 7-134). Median platelet counts for all patients (responders and non-responders) increased over time, exceeding the platelet count thresholds of 30x10 9/L at day 57 and 50x10 9/L at day 120 (Figure 1B). The mean number of weeks with platelet counts ≥50x10 9/L and/or ≥30x10 9/L and doubling from baseline was both 9.3 weeks (SD, 10.1). Three patients (12%) received rescue medication in the main treatment period. Fifteen patients (58%) completed 24 weeks of rilzabrutinib and 11 (42%) entered the LTE.
Over the main treatment period, the median duration of treatment was 167 days (range, 7-169). Sixteen patients (62%) had ≥1 related treatment-emergent adverse event (AE), including 35% diarrhea, 23% headache, and 15% nausea. Most AEs were grade 1 or 2; there was 1 treatment-related AE of grade 3 blood creatinine phosphokinase increase. There was no treatment-related grade ≥2 bleeding/thrombotic events or infections, serious AEs, or deaths.
Conclusion: Part B study results were consistent with part A. Rilzabrutinib demonstrated rapid, stable, and durable platelet responses in patients with relapsed ITP, with a favorable safety profile in part B.
OffLabel Disclosure:
Cooper:Sobi: Honoraria; Sanofi: Honoraria; Novartis: Honoraria, Research Funding; Rigel: Research Funding. Jansen:Erasmus MC: Current Employment; Principia, Argenx, Sobi, Sanofi, CSL Behring: Research Funding; European Patent Office: Patents & Royalties: P133951EP00; Novartis, 3SBIO, Amgen, Sobi: Speakers Bureau; Novartis, Sanofi: Membership on an entity's Board of Directors or advisory committees; 3SBIO, Novartis, Amgen: Other: Travel, accommodations, expenses. Bird:Amgen: Speakers Bureau; Rigel: Research Funding; Principia (Sanofi): Research Funding. Mayer:MSD: Research Funding; Novartis: Research Funding. Sholzberg:CSL Behring: Research Funding; Pfizer: Honoraria, Research Funding; Octapharma: Honoraria, Research Funding. Tarantino:Genentech: Consultancy; Biomarin: Consultancy; Novartis: Consultancy; Octapharma: Consultancy, Other: Clinical trial investigator; Principia: Consultancy; Spark: Other: Clinical trial investigator; Takeda: Other: Clinical trial investigator, Research Funding; Amgen: Consultancy. Garg:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Alnylam: Honoraria, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Janssen: Membership on an entity's Board of Directors or advisory committees. Ypma:Janssen: Honoraria; Janssen: Other: support for attending meetings and/or travel. McDonald:Sobi: Consultancy; Novartis: Consultancy; Amgen: Consultancy; Grifols: Research Funding; Rigel: Research Funding. Percy:Roche-Chugai: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Sobi: Consultancy, Honoraria, Speakers Bureau; LFB: Consultancy, Honoraria, Speakers Bureau; University Hospitals Birmingham NHS Foundation Trust: Current Employment. Košťál:Principia Biopharma Inc, a Sanofi Company: Consultancy. Gernsheimer:Alpine Immune Science: Consultancy. Diab:Sanofi: Current Employment. Yao:Sanofi: Current Employment. Daak:Sanofi: Current Employment. Kuter:Rubius: Current equity holder in publicly-traded company; AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui. Immunovant, Incyte, Inmagenebio: Consultancy; AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui, Immunovant, Incyte, Inmagenebio, Ke: Honoraria; UpToDate: Patents & Royalties: UpToDate Chapters; Platelet Disorder Support Association: Membership on an entity's Board of Directors or advisory committees; Kezar, Kyowa-Kirin, Merck Sharp & Dohme: Honoraria; Kezar, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Nuvig, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Protagonist, Rigel, Sanofi (Bioveratif), Sanofi (Principia), Sanofi (Genzyme), Sobi (Dova), Takeda, UCB, Up-To-Date, Zafge: Consultancy; Alnylam, BioCryst, Novartis, Rigel, Sanofi (Principia), Takeda (Bioverativ), and UCB: Research Funding.
Yes, it is off label. Rilzabrutinib is an investigational therapy being evaluated in a clinical study for the treatment of patients with immune thrombocytopenia.